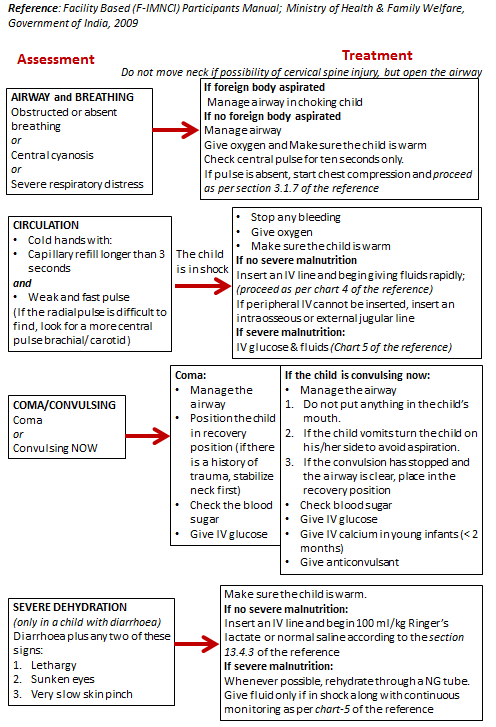
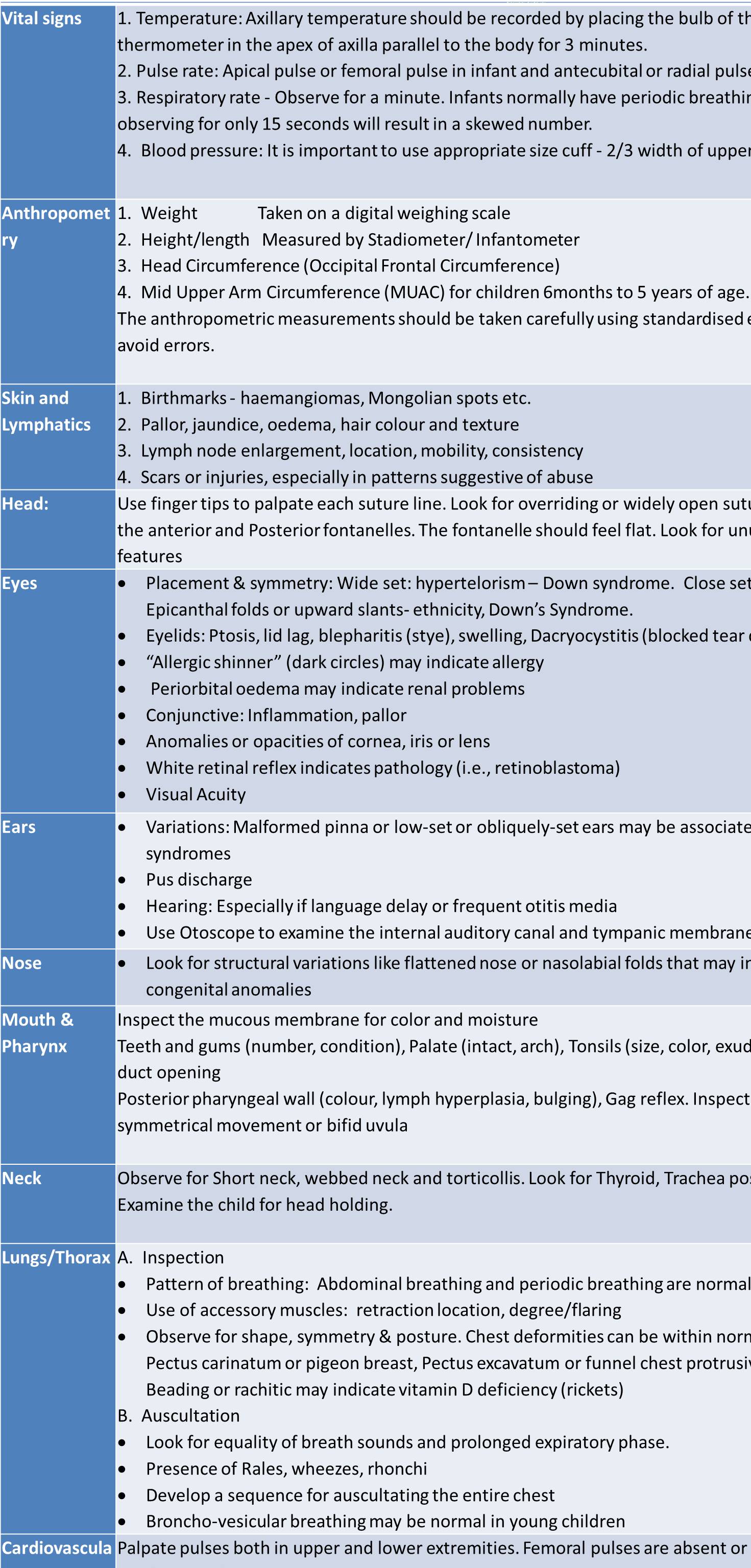
This blog explains the additional, age-specific points that must be covered while taking the history of a pediatric patient, beyond the standard general history format. It guides MBBS students through essential components such as birth history, feeding practices, immunization status, developmental milestones, past illnesses, drug history, family and social environment, and clues to growth or developmental problems. The blog provides a structured, student-friendly approach to ensure that important pediatric details are not missed during case taking.