Type of Vaccine
Live attenuated liquid vaccine
COMPOSITION
Monovalent vaccine containing live attenuated rotavirus derived from a neonatal strain isolated in India
Number of doses per vial
Rotavirus vaccine supplied under UIP has 10 doses per vial.
Identification features
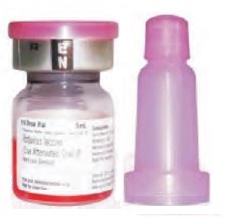
The vaccine is generally a pink liquid but may sometimes change to orange or light yellow in colour. This change in colour does not impact the quality of the vaccine.
The vaccine is supplied with a pink coloured dropper that is longer and wider than the dropper used for OPV.
The vaccine should be administered only with the dropper supplied by the manufacturer
Schedule of immunization under UIP
Under UIP, Rotavirus vaccine is administered at 6, 10 and 14 weeks of age, along with other routine vaccines
For programmatic reasons, the Government of India recommends vaccination upto a maximum of one year of age for BCG, Pentavalent, IPV and Rotavirus.
Dose
5 drops (0.5 ml)
Route of administration
Oral
Reconstitution
Reconstitution is not required as the vaccine is already in liquid form
Does the breastfeeding need to be withheld for some time before or after vaccination?
Breast feeding does not impair the response to rotavirus vaccine. There are no restrictions on the infant’s feed, including breast-milk, either before or after vaccination with Rotavirus Vaccine
Is any other RVV also available in India?
Currently there are three Rotavirus Vaccines licensed and available in market;
1. Rotavac®, a monovalent live vaccine manufactured by Bharat Biotech. This is presently used under UIP and given in 3 doses at ages 6 weeks, 10 weeks and 14 weeks.
2. Rotarix®, a monovalent live vaccine, manufactured by GlaxoSmithKline Biologicals. It is given in 2 doses at ages 2 months and 4 months.
3. RotaTeq®, a Pentavalent live vaccine manufactured by Merck and Co. It is given in 3 doses at 2, 4 and 6 months of age.
Inter-changeability of vaccine manufacturers:
As the composition of the available Rotavirus vaccines and dosage guidelines are different, Rotavirus vaccine from SAME manufacturer is to be used to complete the immunization schedule of an infant.
Contraindications
Contraindications:
1. Known or documented allergic reaction to the vaccine.
2. History of documented intussusception or abdominal surgery or intestinal malformation
3. Known case of immunodeficiency
Rotavirus vaccine administration should be deferred till recovery;
• In infants with moderate or severe acute illness like moderate to severe diarrhea or vomiting and requiring rehydration therapy
However, if the infant has minor illnesses, such as low grade fever and upper respiratory infections (URI), the vaccine can be given.
Is a booster dose recommended?
NO, a booster dose of Rotavirus Vaccine is not recommended because;
• Rotavirus Vaccine largely provides protection for at least first 2 years of life, when the risk of rotavirus diarrhea is highest.
• Also, current scientific evidence suggests that by the second year, most children are exposed to rotavirus and develop protective antibody.
Storage:
At State, Regional and district stores:
The Rotavirus Vaccine should be stored at -20°C, in the walk-in freezers (WIFs) or deep freezers (DFs).
Storage below the district level:
RVV should be stored at +2°C to +8°C, in ice-lined refrigerators (ILRs).
In the ILR, Rotavirus vaccine should be stored at or above BCG level.
Should the droppers also be stored along with the vaccine?
NO, the droppers for administration of the vaccine are to be stored at room temperature as freezing can cause the droppers to crack. The droppers are to be stored and carried along with the other dry supplies (e.g. syringes) outside the vaccine carrier.
Where the Rotavirus vaccine should be kept at the immunization session?
Opened Rotavirus vaccine is to be placed on the ice pack kept outside during the session
Is the vaccine eligible for ‘Open Vial Policy’?
The open vial policy is NOT applicable to Rotavirus vaccine
A rotavirus vaccine vial can be used up to a maximum of 4 hours after opening
Therefore the date and time of opening a rotavirus vaccine vial must be recorded on the vial.
What is the maximum age limit for giving the first dose of Rotavirus vaccine?
Under UIP, the upper age limit for giving the first dose of Rotavirus vaccine is one year of age. However, if the child has received first dose of Rotavirus vaccine by 12 months of age, the course should be completed by giving two more doses at an interval of 4 weeks.
What If the child spits out the Rotavirus vaccine or vomits immediately after having it?
The dose is to be repeated at the same visit
To prevent spitting, the drops are put towards the inner cheek. Avoid putting the drops over the tongue.
What are the common side-effects of Rotavirus vaccine?
Mild and transient symptoms include
• Vomiting
• Diarrhoea
• Cough
• Runny nose
• Fever
• Irritability and
• Rash
Protective efficacy
The available Rotavirus Vaccines are observed to be effective in preventing SEVERE rotavirus diarrhea by 54-60%.
There is also some evidence that Rotavirus vaccination leads to herd protection in unvaccinated older children and adults.
List the severe side effects of pentavalent vaccine which may occur rarely.
A rare adverse event of ‘intussusception’ has been reported after Rotavirus vaccine. The risk of intussusception is about 1–2/100,000 infants vaccinated and occurs shortly after the first dose.
Such children present with severe pain abdomen (excessive crying) and repeated episodes of vomiting, blood in stool and dehydration. Such children need to be referred immediately to hospital for appropriate management
Why is RVV included in the NIS despite medium efficacy and a small risk of intussusception?
• Available evidence suggests that rotavirus vaccines are most effective at preventing the severe and life-threatening cases of rotavirus diarrhea.
• Intussusception can occur in children even without rotavirus vaccine.
• Rotavirus vaccine is already being used in national immunization program of 80 countries.
• There is also some evidence that Rotavirus vaccination leads to herd protection in unvaccinated older children and adults
The risk of adverse events after rotavirus vaccination is much lower than the risk of severe rotavirus disease in unvaccinated children.
Hence, rotavirus vaccine is recommended to prevent rotavirus disease in infants and young children.
Will vaccination with Rotavirus vaccine prevent all diarrheas?
No, it does not prevent all diarrheas.
Diarrhea is caused by many organisms, of which Rotavirus is one of the leading causes in children.
Rotavirus vaccine is effective in preventing diarrhea due to Rotavirus only.
So the child may still get diarrhea due to other infectious and non-infectious causes even after receiving Rotavirus vaccine.
Why was the need felt to introduce Rotavirus vaccine into the NIS?
• Diarrheal diseases are the leading cause of childhood mortality in India and Rotavirus is responsible for nearly 40% of moderate to severe diarrhea in under-five children
• Apart from the burden of death, diarrhea is also an important contributor to long-term nutritional deficiency like stunting, wasting, malnutrition and loss of cognitive development
• Rotavirus is highly contagious and resilient therefore sanitation and hygiene improvements have less impact on transmission of rotavirus diarrhea
• The incidence of rotavirus infection is similar in both developed and developing countries. However, more than 80% of rotavirus deaths occur in developing countries, where poverty, malnutrition and limited access to health services exacerbate the problem.
• It has been observed that rotavirus infects Indian children at an age younger than the children in developed countries. The exposure is still earlier among children in the poorest, rural households with the highest risk of mortality.
• Marked declines have been observed in hospitalizations and deaths due to rotavirus in countries that have introduced rotavirus vaccines under national immunization programmes, e.g. Australia, USA, Mexico and Brazil
Therefore, WHO has recommended inclusion of Rotavirus Vaccines in national immunization programmes of all countries, particularly in South and South-Eastern Asia.
Enumerate other interventions for reducing under- five deaths due to diarrhea.
• Rotavirus vaccination
• Measles vaccination
• Exclusive breastfeeding for 6 months and continued breastfeeding with appropriate complementary feeding
• Vitamin A supplementation in children 6-59 months
• Appropriate management of diarrhea with
o Oral rehydration solution (ORS) and
o Zinc (for 14 days)
• Access to safe drinking Water,
• Access to adequate Sanitation
• Hand washing with soap etc. will contribute in reducing under-five deaths due to diarrhea
What should be done if a child has received one or two doses of Rotavirus vaccine in private facility?
Please remember that there are three Rotavirus vaccines available in the market. These vaccines differ in composition and dosage schedule. The vaccination schedule must be completed using the same vaccine. Please follow the steps mentioned below:
Verify the brand name of the Rotavirus vaccine given on the immunization card. If not written, ask the parents to check with the doctor about the name of the Rotavirus vaccine.
1. If Rotavirus vaccine given in private facility is same as that being given under UIP, then the next doses can be given from govt. facility under UIP at interval of 4 weeks between doses
2. If Rotavirus vaccine given in private facility is NOT the same as that being given under
UIP, then the child should be given a new course of all 3 doses of Rotavirus vaccine under UIP, at interval of 4 weeks between doses.
3. If the parents are not sure about the name of Rotavirus vaccine given in private facility, start a new course with all three doses of Rotavirus vaccine at an interval of 4 weeks.
References:
1. GOI. Immunization Handbook for Medical Officers. New Delhi: Department of Health and Family Welfare; 2016.
2. GOI 2015. Introduction of Rotavirus Vaccine in the UIP, OPERATIONAL GUIDELINES. Immunization Division, Ministry of Health & Family Welfare, New Delhi
3. GOI 2015. Introduction of Rotavirus Vaccine in the UIP, FAQs for Medical Officers. Immunization Division, Ministry of Health & Family Welfare, New Delhi, IndiaIndia
National Immunization Schedule in India; 2017: http://www.ihatepsm.com/blog/national-immunization-schedule-india-2017
Rotavirus vaccine: http://www.ihatepsm.com/blog/rotavirus-vaccine
Pentavalent vaccine: http://www.ihatepsm.com/blog/pentavalent-vaccine
BACILLE CALMETTE GUERIN (BCG) VACCINE: http://www.ihatepsm.com/blog/bacille-calmette-guerin-bcg-vaccine
IPV (INACTIVATED POLIOVIRUS VACCINE): http://www.ihatepsm.com/blog/ipv-inactivated-poliovirus-vaccine
DT & TT Vaccines: http://www.ihatepsm.com/blog/dpt-and-tt-vaccines
Oral Polio Vaccines (OPV): http://www.ihatepsm.com/blog/oral-polio-vaccines-opv
Measles Containing Vaccines (MCV): http://www.ihatepsm.com/blog/measles-containing-vaccines-mcv
HEPATITIS B VACCINE: http://www.ihatepsm.com/blog/hepatitis-b-vaccine
JAPANESE ENCEPHALITIS (JE) VACCINE: http://www.ihatepsm.com/blog/japanese-encephalitis-je-vaccine
Pneumococcal Conjugate Vaccine (PCV): http://www.ihatepsm.com/blog/pneumococcal-conjugate-vaccine-pcv
RABIES VACCINE: http://www.ihatepsm.com/blog/rabies-vaccine
CONCENTRATED VITAMIN A SOLUTION: http://www.ihatepsm.com/blog/concentrated-vitamin-solution
VACCINE VIAL MONITOR (VVM): http://www.ihatepsm.com/blog/vaccine-vial-monitor-vvm
Adverse event following immunization (AEFI): http://www.ihatepsm.com/blog/adverse-event-following-immunization-aefi
Clinical features suggestive of dehydration: http://www.ihatepsm.com/blog/clinical-features-suggestive-dehydration
ORAL REHYDRATION SALT (ORS): http://www.ihatepsm.com/blog/oral-rehydration-salt-ors
Management of Acute Diarrhoea in Children: http://www.ihatepsm.com/blog/management-acute-diarrhoea-children
4 Rules of Home Treatment for Diarrhea in Children: http://www.ihatepsm.com/blog/4-rules-home-treatment-diarrhea-children
Sugar Salt Solution for Rehydration: http://www.ihatepsm.com/blog/sugar-salt-solution-rehydration
6 Steps of ‘Skin Pinch’ Test for Assessing Dehydration: http://www.ihatepsm.com/blog/6-steps-%E2%80%98skin-pinch%E2%80%99-test-a...
Some Clinical features of diarrhea due to common causative organisms: http://www.ihatepsm.com/blog/some-clinical-features-diarrhea-due-common-...
Principles of management of diarrhea: http://www.ihatepsm.com/blog/principles-management-diarrhea
Control of diarrheal diseases: http://www.ihatepsm.com/blog/control-diarrheal-diseases
Indicators of Diarrhea Control: http://www.ihatepsm.com/blog/indicators-diarrhea-control
Vaccines for prevention of diarrhea: http://www.ihatepsm.com/blog/vaccines-prevention-diarrhea
Lecture on acute diarrheal disaeses-1 (epidemiology): https://www.youtube.com/watch?v=8VMSn7mJgm8&t=215s
Lecture on acute diarrheal disaeses-2 (control): https://www.youtube.com/watch?v=85iOg3GNevU&t=41s
Lecture (HINDI) on acute diarrheal disaeses-1 (epidemiology): https://www.youtube.com/watch?v=c-bOaC55u-g&t=5s
Lecture (HINDI) on acute diarrheal disaeses-2 (control): https://www.youtube.com/watch?v=acc-nqkjar4&t=1s
