
Vaccines under UIP, India, 2016
Brief History of the Immunization Program in India
• May 1974:
– Expanded Programme on Immunization (EPI); Launched by WHO
– Included immunization against 6 Vaccine Preventable Diseases (VPD’s)
• Diphtheria,
• Pertussis,
• Tetanus,
• TB,
• Measles and
• Polio
– Later renamed as ‘Universal Child Immunization, 1990
• Jan 1978: EPI launched in India
• Nov 1985: EPI replaced in India by the ‘Universal Immunization Programme (UIP)
• 1992: UIP became a part of Child Survival and Safe Motherhood (CSSM) Program
– NID’s started for polio eradication in 1995
• 1997: UIP became a part of Reproductive and Child Health (RCH) Program
– NPSP launched by WHO and GOI collaboration
• 2005: UIP became a part of NRHM umbrella program
– National Adverse Events Following Immunization (AEFI) Surveillance and Response guidelines launched
– Glass syringes replaced by auto- disable syringes
– The National Technical Advisory Group on Immunization (NTAGI) in India was constituted in 2001, and was reconstituted in 2010
• 2011: National vaccine policy released
• 25th December, 2014 : Mission Indradhanush launched: this seeks to drive towards 90% full immunization coverage of India and sustain the same by year 2020.
Introduction of new antigens in UIP in India (till 2016)
• 1999: MMR was introduced in state immunization program of Delhi as a single dose administered between 15-18 months of age
– MMR now a part of State immunization schedules of only Goa, Puducherry, Sikkim and Delhi
• 2002: Hepatitis B added in 33 Stated ; All over the country by 2011
• 2006: Japanese Encephalitis (JE) added: covered all the 112 endemic districts by – 2010
• 2010: second dose of measles introduced (given with DPT first booster)
• 2011: Hib added in Kerala and Tamil Nadu as ‘Pentavalent vaccine’(Hepatitis B, Diphtheria + Pertussis + Tetanus (DPT) and Haemophilus influenza b (Hib))
• Presently, Pentavalent vaccine has replaced the primary doses of DPT and Hepatitis B all over the country
• 2015: As a part of Polio end game strategy, Injectable Polio Vaccine (IPV) vaccine has been introduced in 6 states from 30th November 2015 in Phase1.
– In 2016, IPV was introduced in the remaining states
• Rotavirus Vaccine: to be given under UIP as a 3 dose vaccine along with DPT 1st, 2nd and 3rd dose in a phased manner, initially in four states i.e.
– Andhra Pradesh,
– Odisha,
– Haryana and
– Himachal Pradesh in first quarter of 2016.
– Subsequently, the vaccine will be scaled up in entire country.
National Immunization schedule
• For Pregnant Women

• For Infants & Children till Recently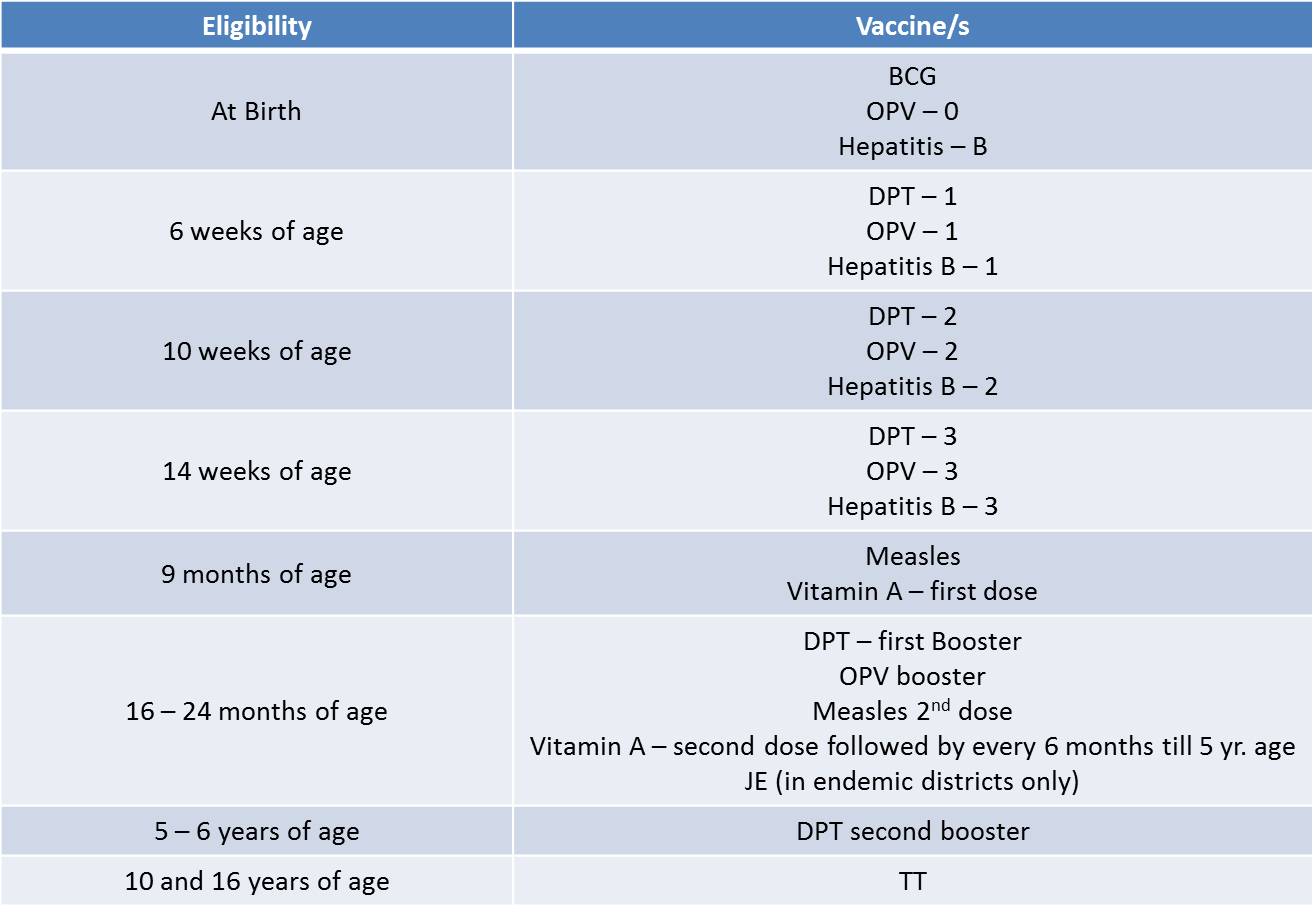
• For Infants & Children as in 2016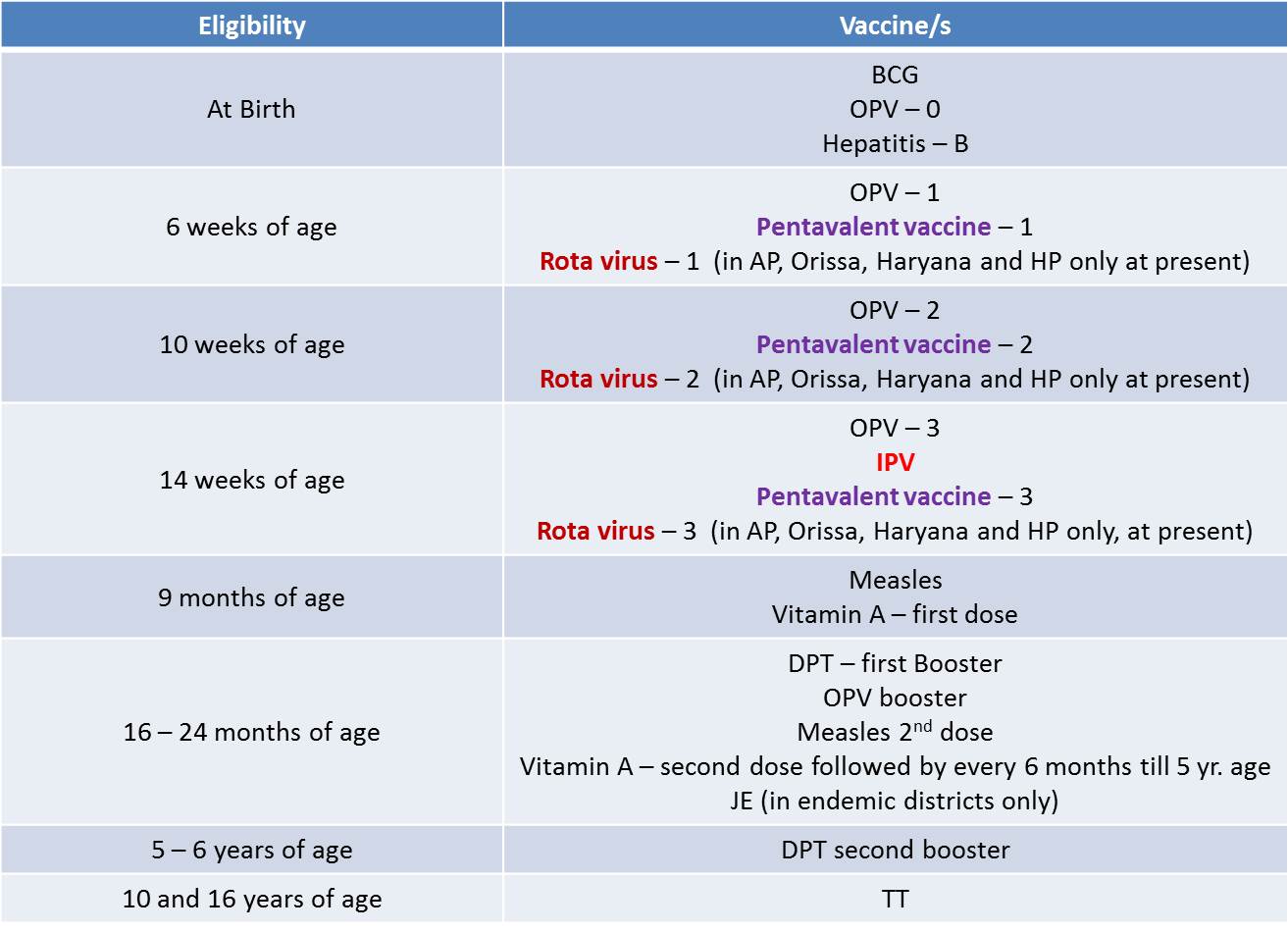
New vaccines to be introduced as per National Technical Advisory Group on Immunization (NTAGI) recommendation
1. Rota virus vaccine: NTAGI recommended the introduction of rotavirus vaccine in Universal Immunization Programme in a phased manner.
2. Rubella vaccine is to be introduced as MR vaccine replacing the measles containing vaccine first dose (MCV1) at 9 months and second dose (MCV2) at 16-24 months.
References
• Chandrakant Lahariya. A brief history of vaccines & vaccination in India; Indian J Med Res. 2014 Apr; 139(4): 491–511
• Park’s Textbook of PSM
• National Health Mission, MoHFW: available at: http://nrhm.gov.in/nrhm-components/rmnch-a/immunization/background.html last accessed on 9th Nov 2016
• UIP, Directorate of Family Welfare, Govt of Delhi: available at http://delhigovt.nic.in/dept/health/dfw/universal_program.htm last accessed on 9/11/2016
for vaccination schedule in India, 2016: http://www.ihatepsm.com/blog/vaccination-schedule-india-2016
MDG Goal 4 achievement in India, 2016: http://www.ihatepsm.com/blog/millenium-development-goal-no4-achievement-...
