Measles containing vaccines are available either as monovalent measles vaccine or in combination with rubella (MR) or mumps and rubella (MMR) vaccines and with mumps, rubella and varicella (MMRV) vaccine.
Measles, MR and MMR have been used under NIS. Presently, MR vaccine is to be made available under routine immunization across the country.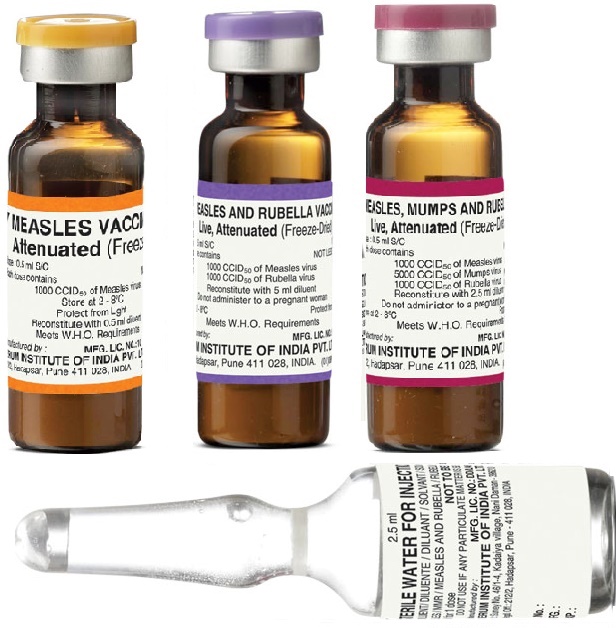
Identification features
The measles containing vaccines are available in dark glass bottles (Fig. 4.9) to protect from sunlight, but the size of bottle is longer than the BCG vial.
Diluent
Distilled water
Which strain of individual viruses is used?
• Edmonston-Zagreb strain of measles virus
• L-Zagreb strain of mumps virus
• Wistar RA 27/3 strain of Rubella virus
COMPOSITION
The reconstituted MCVs contain in a single dose of 0.5 ml:
• Not less than 1000 CCID50 of Measles virus (Measles, MR and MMR)
• Not less than 1000 CCID50 of Rubella virus (MR, MMR)
• Not less than 5000 CCID50 of Mumps virus (MMR)
Dose
0.5 mL
Route of administration
Subcutaneous (S/C)
Note: the vaccines are effective through intramuscular route also
Site of injection
Right upper arm
Type of vaccine
Live attenuated
Schedule under NIS
MR vaccine is be administered in two doses; replacing the measles vaccine.
• The first dose is to be given between 9 and 12 months of age and
• The second dose is to be given at 16-24 months of age
During an epidemic it can be given at 6 months of age also but not to be counted and should be repeated at the usual age of 9 months.
Side effects
Adverse reactions are generally mild and transient:
• Slight pain and tenderness at the site of injection
• High fever (>39.4°C) after 7–12 days after vaccination; which may occasionally (1 / 3000) induce febrile seizures;
• Transient rash in about 2% of vaccinated children
These may last for 1-3 days.
Some rare but serious adverse reactions:
• Thrombocytopenic purpura in approximately 1 in 30 000 vaccinated individuals
• Anaphylaxis; very rare (1 in 1 million)
• Arthralgia when given in adolescents or adults
Contraindications
• High fever (>102 °F / 38–39 °C) or serious disease
• Pregnancy
• History of an anaphylactic reaction to neomycin, gelatin or other components of the MR vaccine
• Severely immune-compromised e.g. congenital, HIV infection (full blown AIDS), advanced leukemia or lymphoma, treatment with high-dose steroids or antimetabolites
• Administration of immunoglobulins or other antibody-containing blood products (as may interfere with the immune response to the vaccine)
o Delay vaccination for 3 – 11 months after blood or blood products administration
o Avoid such blood products for 2 weeks after MR vaccination
Protective efficacy and duration
Measles: It is 85% when given at 9 months and 95% when given at >12 months of age.
Rubella vaccine is even more efficacious than measles, (appx. 95% when given at 9 months and >99% when given after 1 year).
Efficacy may last lifelong.
Storage of vaccine
• The vaccine can be stored for a long term , e.g.,at PHC -15°C to -20°C, in the deep freezer.
• The MR vaccine is very sensitive and should always be protected from sunlight.
• The vaccine must be stored between 2°C and 8°C for a few weeks at the place of use such as the subcenter.
• The diluent should also be stored at +2°C to +8°C.
• The reconstituted vaccine should be kept on ice and used within 4 hours.
Why should the reconstituted measles vaccine not be used after 4 hours of opening?
The vaccine after reconstitution is vulnerable to contamination which can lead to toxic shock syndrome (TSS) in the recipient.
The vaccine is vulnerable to contamination as there is no preservative added to this live vaccine.
Postexposure prophylaxis for Measles
The vaccine may be given, within 3 days of contact (72 hours), in previously unvaccinated children >9–12 months old. The immunity develops 10–12 days after vaccination.
Enumerate some complications of Measles infection in children.
1. Diarrhea
2. Pneumonia
3. Otitis media
4. Encephalitis
5. Reactivation of pulmonary TB
6. Acute deficiency of Vitamin A, may even proceed to Keratomalacia
7. Subacute sclerosing pan-encephalitis (SSPE)
What is subacute sclerosing pan-encephalitis (SSPE)?
SSPE is a rare complication of measles infection, which develops many years after the initial infection.
It is characterized by progressive mental deterioration leading to paralysis, involuntary movements, muscle rigidity and coma. There is no cure and it is usually fatal in 1 – 3 years after onset.
It occurs probably due to persistence of the virus in the brain.
Why is MR/MMR vaccine contraindicated in pregnant women?
There may be a risk of the fetus developing congenital rubella syndrome (CRS). In fact a woman must be counseled to avoid pregnancy for 3 months after vaccination for rubella.
What is the most serious complication of rubella infection?
CRS which may occur in the newborn if the rubella infects a pregnant woman is the most serious complication.
What is CRS?
CRS, (Congenital Rubella syndrome) is a set of serious congenital defects a child may be born with when a pregnant women gets Rubella infection in early pregnancy, causing
• Blindness (congenital cataract)
• Deafness
• Heart defects
• Mental retardation
• Liver disorders and
• Other hematological disorder, incompatible with normal living.
Does the vaccine protect against sub-acute sclerosing pan-encephalitis (SSPE) and CRS too?
The virtual disappearance of sub-acute sclerosing pan encephalitis (SSPE) and CRS in countries where measles and rubella have been eliminated strongly suggests that the vaccine protects against
• SSPE by preventing measles infection and
• CRS by preventing rubella infection during pregnancy
Does a child need to be vaccinated if she or he has history of any fever-rash illness including measles or rubella disease?
Yes, every child must be vaccinated with two doses, as per the national immunization schedule with MR vaccine at the recommended ages, irrespective of any past fever-rash illness or measles/rubella disease.
If a child has received the Measles Rubella vaccine before 9 months of age, is it necessary to repeat the vaccine later?
Yes, the Measles Rubella vaccine should be administered, according to the National
Immunization Schedule i.e. at completion of 9 months and at 16-24 months
As measles and JE vaccine doses are recommended for the same age group, can they be given together?
Yes, two live injectable vaccines can be administered simultaneously at different sites, otherwise at a minimum interval of 28 days.
What is the recommendation regarding measles vaccination in HIV positive individuals?
Given the severe course of measles in patients with advanced HIV infection, routine vaccination with measles is recommended for the susceptible and asymptomatic HIV-positive children and adults.
For those who are symptomatic but not severely immunosuppressed, vaccination may still be considered.
References:
1. GOI. Immunization Handbook for Medical Officers. New Delhi: Department of Health and Family Welfare; 2016.
2. Park K. Principles of epidemiology and epidemiologic methods. In: Park's Textbook of Preventive and Social Medicine, 24th ed. Jabalpur, India: M/S Banarasidas Bhanot Publishers; 2017.
3. Park K. Epidemiology of communicable diseases. In: Park's Textbook of Preventive and Social Medicine, 24th ed. Jabalpur, India: M/S BanarasidasBhanot Publishers; 2017.
4. GOI 2017. Introduction of MR Vaccine; National Operational Guidelines: Ministry of Health and Family Welfare, New Delhi
5. MR vaccination campaign; some common concerns of parents. Ministry of Health and Family Welfare website. Available at: http://mohfw.nic.in/sites/default/files/Measles-Rubella.pdf accessed on 28th October 28, 2017
6. PRODUCTS SUPPLIED IN INDIA. Serum Institute of India website. Available at: http://www.seruminstitute.com/product_overseas.php accessed on 1st November 2017
National Immunization Schedule in India; 2017: http://www.ihatepsm.com/blog/national-immunization-schedule-india-2017
Rotavirus vaccine: http://www.ihatepsm.com/blog/rotavirus-vaccine
Pentavalent vaccine: http://www.ihatepsm.com/blog/pentavalent-vaccine
BACILLE CALMETTE GUERIN (BCG) VACCINE: http://www.ihatepsm.com/blog/bacille-calmette-guerin-bcg-vaccine
IPV (INACTIVATED POLIOVIRUS VACCINE): http://www.ihatepsm.com/blog/ipv-inactivated-poliovirus-vaccine
DT & TT Vaccines: http://www.ihatepsm.com/blog/dpt-and-tt-vaccines
Oral Polio Vaccines (OPV): http://www.ihatepsm.com/blog/oral-polio-vaccines-opv
Measles Containing Vaccines (MCV): http://www.ihatepsm.com/blog/measles-containing-vaccines-mcv
HEPATITIS B VACCINE: http://www.ihatepsm.com/blog/hepatitis-b-vaccine
JAPANESE ENCEPHALITIS (JE) VACCINE: http://www.ihatepsm.com/blog/japanese-encephalitis-je-vaccine
Pneumococcal Conjugate Vaccine (PCV): http://www.ihatepsm.com/blog/pneumococcal-conjugate-vaccine-pcv
RABIES VACCINE: http://www.ihatepsm.com/blog/rabies-vaccine
CONCENTRATED VITAMIN A SOLUTION: http://www.ihatepsm.com/blog/concentrated-vitamin-solution
VACCINE VIAL MONITOR (VVM): http://www.ihatepsm.com/blog/vaccine-vial-monitor-vvm
Adverse event following immunization (AEFI): http://www.ihatepsm.com/blog/adverse-event-following-immunization-aefi
