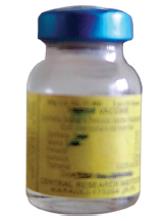
Diphtheria, Pertussis, and Tetanus(DPT; Triple Antigen)
Identification features
The vaccine is available in small glass bottles mostly having a blue metallic cover.
Type of vaccine
Killed (diphtheria toxoid, tetanus toxoid, and killed bacteria of pertussis)
Site of injection
Anterolateral aspect of LEFT mid-thigh
Schedule for immunization under UIP
Two DPT boosters are to be given at:
1. 16– 24 months and
2. 5–7 years
(For primary immunization under UIP, Pentavalent – 1, 2 and 3 given at 6, 10, and 14 weeks has replaced DPT – 1, 2 and 3)
Contraindication/s to DPT vaccine
• Serious illness requiring hospital admission
• Presence of a progressive neurological disorder
• Convulsions
• A severe reaction to one of the previously given DPT, e.g.,
- Shock or collapse
- Persistent crying episode
- Anaphylactic reaction
• High temperature: >40°C (104°F)
• Other neurological manifestations
Protective efficacy
Diphtheria: Almost 100%
Tetanus: Almost 100%
Pertussis: 75%
How will you manage the contacts of a Diphtheria patient?
1. All contacts should have a throat swab culture.
2. Determine their immunity status:
• Primary doses/booster received within previous two years: No further action
• If primary course/booster dose received more than 2 years before: Only a booster dose of diphtheria toxoid needs be given
• Non – immunized:
o Prophylactic penicillin or erythromycin
o Diphtheria antitoxin, 1000 – 2000 units
o Active immunization against diphtheria with two doses of diphtheria toxoid
3. Daily examination for evidence of diphtheria for at least a week after exposure
4. Throat swab culture weekly for several weeks
How will you manage the contacts of a pertussis (whooping cough) patient?
Prophylactic erythromycin or ampicillin for 10 days.
Is the pertussis vaccine given to the adults same as that given to young children?
Adults should be given only acellular pertussis vaccine 1, 3, 4
Tetanus Toxoid (TT)
Identification features
The vaccine is available in small glass bottles having a golden yellow metallic cover.
Site of injection
Upper arm
Type of vaccine
Toxoid (killed)
Schedule for immunization
Under NIS
For pregnant female: First dose (TT-1) early in pregnancy and the second dose (TT-2) 4 weeks after the first dose. (If she has had two doses of TT in her last pregnancy within last 3 years, only a single dose is to be given and this is called as a "booster dose")
TT-2 or the booster dose is to be given preferably before 36 weeks of pregnancy. However, these should be given even if she reports after 36 weeks have passed. TT should also be given to a woman in labor, if she has not previously received TT.
For children: A booster dose each at 10 and 16 years of age.
After an injury
• Immunity category A (received complete course of TT or booster within past 5 years)
- No action
• Immunity category B (complete course/ booster >5 years but <10 years ago)
- Single dose of TT
• Immunity category C (complete course/ booster >10 years ago)
- Single TT if clean wound
- Single TT with human tetanus immunoglobulin if wound not clean
• Immunity category D (not immunized or immunity status unsure)
- Two doses of TT 1-2 months apart (nothing else if wound clean).
- Two doses of TT as given in the preceding text and human tetanus immunoglobulin if wound not clean.
Is tetanus a contagious disease?
No
What are the "5 cleans”?
These are the recommendations for ensuring a clean delivery in order to prevent neonatal tetanus. These include the following:
1. Clean hands and fingernails
2. Clean delivery surface
3. Clean blade to cut the cord
4. Clean cord tie and
5. Clean cord, i.e., no application on the cord stump
What is the level of tetanus antitoxin in blood which is considered protective?
0.01 IU/mL
Name the three criteria which are used to classify the districts in India into various neonatal tetanus (NT) risk categories.
These are as follows:
1. 1 Incidence rate of NT
2. Immunization coverage in pregnant women with TT-2/booster dose
3. Proportion of clean deliveries attended by trained birth attendants 3
Note: The color coding of the metallic cap, i.e. blue for DPT, green for DT, and golden for TT, has not remained very strict recently. Many manufacturers use color which is different from the previously recommended ones. Hence most likely the name of the vaccine will not be concealed while being put up for spotting.
FACTS COMMON FOR DPT AND TT VACCINES
Whether part of NIS?
Yes
Dose
0.5 mL
Route of administration
Deep intramuscular (I/M)
Preparation of site of administration
Clean with spirit
Instruction to mother after vaccination
To give paracetamol in case of fever.
Protective efficacy
Pertussis—80%
Diphtheria and tetanus—almost 100%
Diluent
DPT, DT, and TT are available as liquid suspension and are to be injected as such. Hence no diluent is needed.
Side effects of DPT/DT/TT
• Fever
• Local swelling and pain
• Neurological: Encephalitis, convulsions, infantile spasms, Reye's syndrome (rare, most likely due to the pertussis component)
For the fever and local reactions, paracetamolis given (mostly a prophylactic dose is given to each child following vaccination with DPT).
Storage
Between 2°C and 8°C
DPT, DT, and TT should be stored at a temperature between 2°C and 8°C.
They MUST NEVER BE FROZEN.
When issued to a subcenter, it should be used within a week.
Freezing damages the vaccine and
• Considerably reduces the potency of toxoid.
• Induces severe side effects if injected. This is because it damages the physical structure of the toxoid particles which results in more side effects.1, 2, 3
How can we make out whether the given DPT, DT, TT, Pentavalent vaccine or Hepatitis-B vaccine vial has been frozen in past?
By performing "Shake test”
What is "Shake test”?
Shake test is used for determining if an adsorbed vaccine has been frozen anytime in the past. The test is valid for "adsorbed vaccines” only, such as DPT, DT, TT, Pentavalent vaccine and Hepatitis-B.
Steps of "shake test”
1. Take the suspected freeze damaged vial.
2. Take another vial of the same vaccine from the same batch and the same manufacturer and label it is as "control.”
3. Deliberately freeze the "control” and then thaw it at room temperature.
4. Shake both the vials vigorously.
5. Place both of them on a flat surface, side by side, against a source of light.
6. Observe and compare the sedimentation rate in both the bottles.
7. If the TEST vial sediments slower than the FROZEN vial then use the vaccine.
8. If the sedimentation is similar in both the vials OR the TEST vial sediments faster than the FROZEN vial then the vaccine is damaged, discard it.1, 2

Figure.1 Both the vials are vigorously shaken and kept side by side—the starting point.

Figure 2 First test vial.

Figure 3 Second test vial.
(Tip to remember in shake test: "Haste makes waste"—faster sedimentation indicates damage. Discard the vial.)
IPV is also freeze sensitive, is ‘shake test’ applicable to IPV too?
NO, shake test is not valid for determining if IPV vial has been frozen anytime. This is so because IPV does not contain aluminum adjuvant1.
What is the adjuvant in DPT vaccine?
DPT is adsorbed on a mineral carrier like aluminum phosphate or hydroxide which acts as adjuvant—it is the preferred preparation of choice.4
What is the role of pertussis component in DPT?
In addition to immunizing against whooping cough, the pertussis component also enhances the potency of diphtheria toxoid.3
References:
1. GOI. Immunization Handbook for Medical Officers. New Delhi: Department of Health and Family Welfare; 2016.
2. Park K. Principles of epidemiology and epidemiologic methods. In: Park's Textbook of Preventive and Social Medicine, 24th ed. Jabalpur, India: M/S Banarasidas Bhanot Publishers; 2009.
3. Park K. Epidemiology of communicable diseases. In: Park's Textbook of Preventive and Social Medicine, 24th ed. Jabalpur, India: M/S BanarasidasBhanot Publishers; 2009.
4. Triple antigen; Serum Institute of India Limited. Available at: http://www.seruminstitute.com/ content/products/product_triple_antigen.htm. Accessed December 5, 2011.
National Immunization Schedule in India; 2017: http://www.ihatepsm.com/blog/national-immunization-schedule-india-2017
Rotavirus vaccine: http://www.ihatepsm.com/blog/rotavirus-vaccine
Pentavalent vaccine: http://www.ihatepsm.com/blog/pentavalent-vaccine
BACILLE CALMETTE GUERIN (BCG) VACCINE: http://www.ihatepsm.com/blog/bacille-calmette-guerin-bcg-vaccine
IPV (INACTIVATED POLIOVIRUS VACCINE): http://www.ihatepsm.com/blog/ipv-inactivated-poliovirus-vaccine
DT & TT Vaccines: http://www.ihatepsm.com/blog/dpt-and-tt-vaccines
Oral Polio Vaccines (OPV): http://www.ihatepsm.com/blog/oral-polio-vaccines-opv
Measles Containing Vaccines (MCV): http://www.ihatepsm.com/blog/measles-containing-vaccines-mcv
HEPATITIS B VACCINE: http://www.ihatepsm.com/blog/hepatitis-b-vaccine
JAPANESE ENCEPHALITIS (JE) VACCINE: http://www.ihatepsm.com/blog/japanese-encephalitis-je-vaccine
Pneumococcal Conjugate Vaccine (PCV): http://www.ihatepsm.com/blog/pneumococcal-conjugate-vaccine-pcv
RABIES VACCINE: http://www.ihatepsm.com/blog/rabies-vaccine
CONCENTRATED VITAMIN A SOLUTION: http://www.ihatepsm.com/blog/concentrated-vitamin-solution
VACCINE VIAL MONITOR (VVM): http://www.ihatepsm.com/blog/vaccine-vial-monitor-vvm
Adverse event following immunization (AEFI): http://www.ihatepsm.com/blog/adverse-event-following-immunization-aefi
