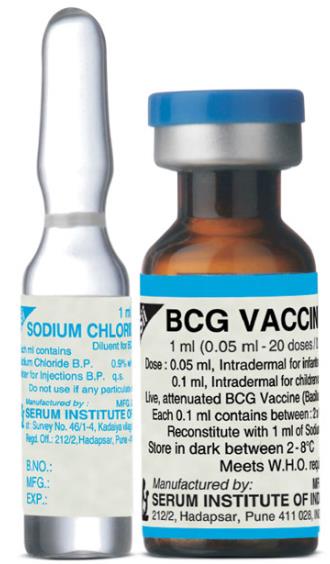
Identification features of BCG vaccine (Fig. 4.1)
• The vaccine is available in dark bottles to protect from sunlight.
• Some bottles have a long neck as the vaccine is packed in vacuum.
Type of vaccine
• Live
• Freeze dried powder
Dose
For institutional deliveries: 0.05 mL at the time of birth or up to age of 4 weeks
• In case of home delivery, if given at 6 weeks: 0.1 mL (along with Pentavalent – 1 and other routine vaccines)
Route of administration
Strictly intradermal injection
Site of injection
Just above the insertion of deltoid on the left arm to maintain uniformity and for helping surveyors in verifying the receipt of the vaccine.
If the injection is too high, too anterior or posterior, the adjacent lymph nodes may get involved, inflamed, and tender.
Schedule of immunization
Single dose at birth.
If not given at birth, give at 6 weeks along with routine vaccination at 6 weeks of age.
Precaution while injecting BCG
• Injection should remain intradermal.
• Do not use antiseptic before injection.
• If spirit has been applied, wait for it to completely dry off before injecting the vaccine.
• For 6 months, no injection should be given in the same arm in which BCG was given.
What is the reason for giving BCG through strictly intradermal route? What if the dose goes deeper, e.g., subcutaneous?
If BCG vaccine penetrates into the deeper tissue, it can cause local abscess and enlarged and painful axillary lymph nodes. There is a small chance of this infection getting disseminated too, if the cellular immunity of the recipient is low.
Why do we give 0.05 mL dose of BCG to newborns (below 1 month of age)?
This is because the skin of newborns is thin and an intradermal injection of 0.1 mL may break the skin and penetrate into the deeper tissue and this may cause local abscess and enlarged axillary lymph nodes.
Which strain is used?
Danish 1331 is the WHO recommended strain of bacteria for the production of vaccine.
Reconstitution
Normal saline is the recommended diluent.
The reconstituted vaccine may be used up to 3 hours and left over should be discarded.
Why cannot we use distilled water as diluent when normal saline is not available?
Distilled water is an irritant to the skin. As BCG is to be given intradermally, distilled water can cause irritation of the skin which may cause ulceration at the site of injection and in severe cases even sloughing of the skin.
Complications
• Prolonged ulceration at the vaccination site
• Severe ulceration
• Suppurative lymphadenitis
• Tubercular osteomyelitis
• Disseminated BCG infection (BCG-osis) and even death
Storage of vaccine
• Long-term storage, e.g., at PHC15°C to20°C, in the deep freezer
• The vaccine may be stored between 2°C to 8°C for a few weeks at the place of use such as the subcenter
• Protect from light
Protective efficacy
Approximately 15–20 years and the protection is partial protection. It is believed that BCG may offer only partial protection from TB but it seems to prevent severe form of childhood tuberculosis such as miliary tuberculosis and tubercular meningitis.
Normal reaction after BCG vaccination
• Immediately after a satisfactory injection— wheal of 5 mm in diameter which later subsides.
• After 2–3 weeks—a papule develops which gradually grows in size till 4-8 mm.
• After 5 weeks—the papule subsides or breaks down into a shallow ulcer, usually covered with a crust.
• Within 6–12 weeks—healing occurs, leaving a permanent scar, 4–8 mm in diameter.
• Mantoux positivity develops usually after8weeksof injection.
If no scar appears after administering BCG, should one re-vaccinate the child?
There is no need to re-vaccinate the child even if there is no scar.
Contraindications to BCG vaccination
• Generalized eczema
• Infective dermatosis
• Pregnancy
• States of reduced immunity (immunocompromised) such as
- Hypogammaglobulinemia
- HIV positive with symptoms
- Congenital immunodeficiency
- Leukemia
- Lymphoma
- Generalized malignancy under chemo-therapy
Which type of immunity is mainly induced by giving BCG vaccine?
BCG vaccine predominantly induces cell mediated (delayed-type hypersensitivity) type of immunity
References:
1. GOI. Immunization Handbook for Medical Officers. New Delhi: Department of Health and Family Welfare; 2016.
2. Park K. Principles of epidemiology and epidemiologic methods. In: Park's Textbook of Preventive and Social Medicine, 24th ed. Jabalpur, India: M/S Banarasidas Bhanot Publishers; 2009.
3. Park K. Epidemiology of communicable diseases. In: Park's Textbook of Preventive and Social Medicine, 24th ed. Jabalpur, India: M/S BanarasidasBhanot Publishers; 2009.
4. Fine PEM, Sterne JAC, Pönnighaus JM, Rees RJW. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 1994;344:1245–9
National Immunization Schedule in India; 2017: http://www.ihatepsm.com/blog/national-immunization-schedule-india-2017
Rotavirus vaccine: http://www.ihatepsm.com/blog/rotavirus-vaccine
Pentavalent vaccine: http://www.ihatepsm.com/blog/pentavalent-vaccine
BACILLE CALMETTE GUERIN (BCG) VACCINE: http://www.ihatepsm.com/blog/bacille-calmette-guerin-bcg-vaccine
IPV (INACTIVATED POLIOVIRUS VACCINE): http://www.ihatepsm.com/blog/ipv-inactivated-poliovirus-vaccine
DT & TT Vaccines: http://www.ihatepsm.com/blog/dpt-and-tt-vaccines
Oral Polio Vaccines (OPV): http://www.ihatepsm.com/blog/oral-polio-vaccines-opv
Measles Containing Vaccines (MCV): http://www.ihatepsm.com/blog/measles-containing-vaccines-mcv
HEPATITIS B VACCINE: http://www.ihatepsm.com/blog/hepatitis-b-vaccine
JAPANESE ENCEPHALITIS (JE) VACCINE: http://www.ihatepsm.com/blog/japanese-encephalitis-je-vaccine
Pneumococcal Conjugate Vaccine (PCV): http://www.ihatepsm.com/blog/pneumococcal-conjugate-vaccine-pcv
RABIES VACCINE: http://www.ihatepsm.com/blog/rabies-vaccine
CONCENTRATED VITAMIN A SOLUTION: http://www.ihatepsm.com/blog/concentrated-vitamin-solution
VACCINE VIAL MONITOR (VVM): http://www.ihatepsm.com/blog/vaccine-vial-monitor-vvm
Adverse event following immunization (AEFI): http://www.ihatepsm.com/blog/adverse-event-following-immunization-aefi
Open Vial Policy: https://ihatepsm.com/blog/open-vial-policy
